Last week we saw how crossbreeding — or more accurately, random matings and/or matings between unrelated populations — do not change gene and genotypic frequencies from generation to generation. This phenomenon is known as the Hardy-Weinberg Equlibrium, named after its co-discovers the English mathematician Godfrey Hardy and the German obstetrician-gynecologist Wilhelm Weinberg.
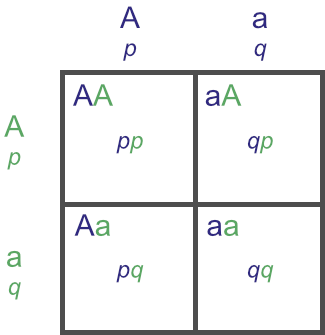
The Hardy-Weinberg Equilbrium states that gene and genotypic frequencies will not change from generation to generation, assuming random matings in the absence of external forces. It further states that, given two alleles at a locus with gene frequencies p and q within a population, that the genotypic frequencies of those alleles will be P = p2, H = 2pq, and Q = q2.
(This second statement is more clear with the following Punnet square:
Remember that p and q are the gene frequencies of the ‘A’ and ‘a’ alleles respectively. P, H and Q are the genotypic frequencies of the ‘AA’, ‘Aa’ and ‘aa’ genotypes respectively. Thus:
P (or ‘AA’) = p × p = p2,
H (or ‘Aa’) = (p × q) + (q × p) = (pq) + (qp) = (pq) + (pq) = 2 × (pq) = 2pq, and
Q (or ‘aa’) = q × q = q2.)
Let’s go back to the first statement, that gene and genotypic frequencies will not change from generation to generation, and revisit the qualifying part: ‘assuming random matings in the absence of external forces’.
What are the external forces that affect a Hardy-Weinberg Equilibrium? The following are all known, and apply to both wild populations and breeding programmes:
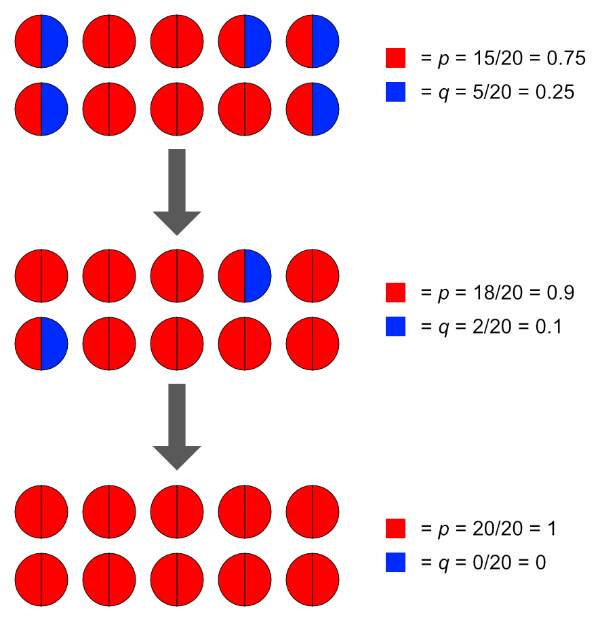
Selection. We’ve shown in this post how selecting for particular alleles changes their frequencies within a population. An example in a breeding programme would be the selection for polled cattle, where it’s desirable to increase the frequency of the ‘P’ (polled, or hornless) allele to 1 (100%). A classic example in nature is the evolution of the peppered moth in Manchester, UK, during the Industrial Revolution. Prior to this period, peppered moths were white-bodied with a ‘peppering’ of brown spots and splotches all over. This colouring was an effective camouflage against tree trunks in a clean environment. Sometime after 1811 however, a new, predominant form arose that was solid brown. This colouring was a more effective camouflage against the soot-covered trunks in polluted areas.
Gene mutations. This is a very rare event, but changes during DNA replication can alter genes and create new alleles. The mere presence of a new allele in a population is enough to shift gene and genotypic frequencies accordingly. One example of this is the explosion of budgerigar colour mutations from the late 1800s on — where once all pet budgies had the same green and yellow and blue markings, there is now a plethora of colours. The popularity of this little Australian parrot led to a population increase worldwide, which increased the chances of rare mutations affecting colours and markings appearing. Breeders would then deliberately select for these, ultimately increasing the gene and genotypic frequencies throughout the worldwide population of budgerigars. Likewise, the many colour varieties of the North American corn snake similarly arose from selective breeding that deliberately shifted frequencies away from those in the wild population.
Migration in and out of a population. The movement of animals in and out of a population can change frequencies significantly, especially if large numbers of very different animals are introduced. An example was briefly mentioned here, where in the 1990s, the introduction to Australia of alpacas with greater density and coverage changed the appearance of the national herd in just a few years.
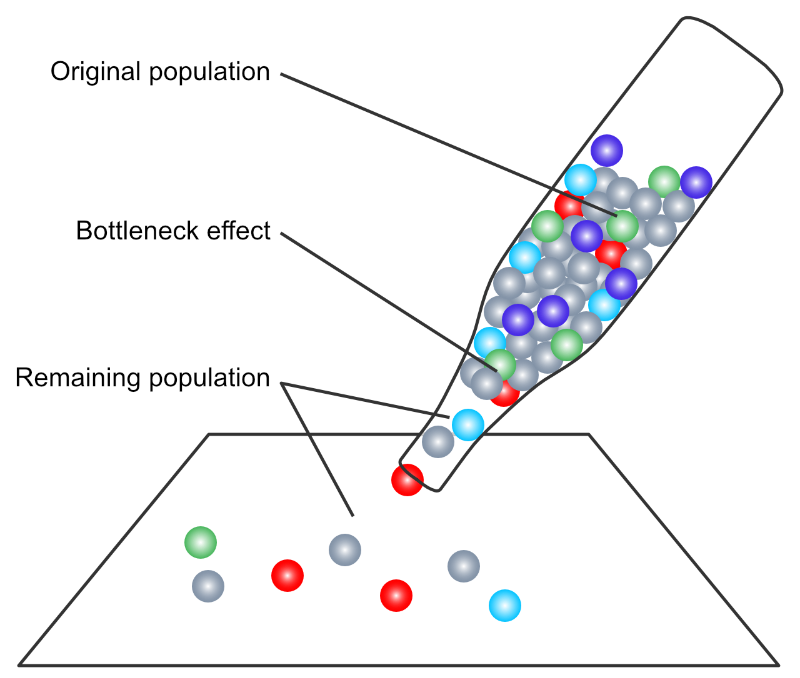
Small population size. Small populations quickly become inbred, and inbred populations, as shown here have altered gene and genotypic frequencies through increased homozygosity. The phenomenon of random genetic drift, more pronounced in small populations, will also affect frequencies. Random drift is where, through the sheer randomness of gene segregation during meiosis, a low frequency (rare) allele may or may not pass from a parent to an offspring. Drift can result in an allele disappearing completely from the population, or actually becoming more common in the population, even to the point of being permanently fixed at the locus. Either event will affect gene and genotypic frequencies in that population. (We’ll cover random genetic drift next week.)
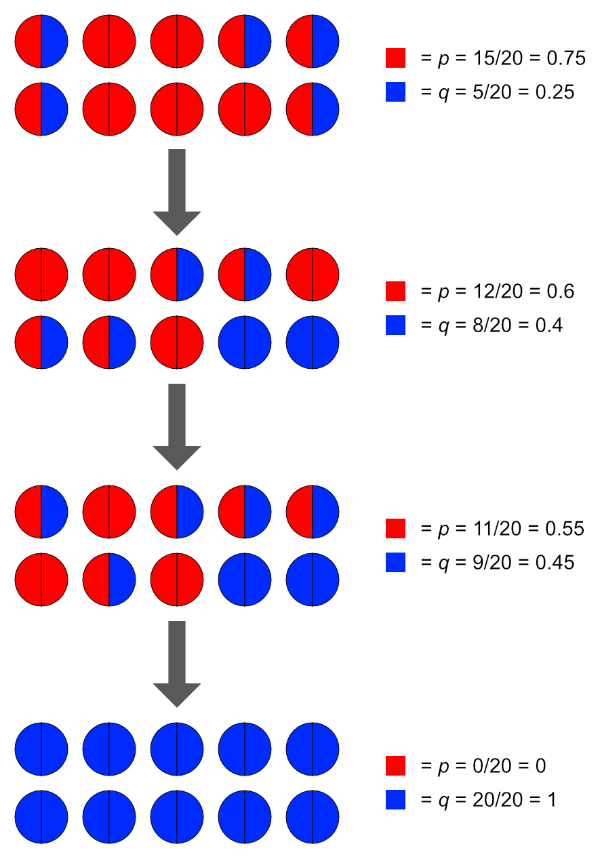
Non-random matings. Random matings will preserve gene and genotypic frequencies from generation to generation as they are simply a vehicle for the genes that randomly assort through meiosis. Non-random matings however are, by definition, subject to external forces. These external forces disrupt the natural randomness of gene assortment that would otherwise occur, and frequencies shift as a result. Allowing only matings between ‘aa’ recessive genotypes, for example, will quickly change the genotypic frequencies at that locus within a population. For example, the recessive ’ss’ genotype in alpacas produces the huacaya (woolly, sheep-like) look rather than the dominant ‘Ss’ or ‘Ss’ suri (dreadlocked, mohair-like) appearance. Huacayas outnumber by far the suri type, worldwide, for several reasons including their popularity and easier wool-processing properties.
Conversely, just one generation of random matings is enough to restore the Hardy-Weinberg equlibrium, as was shown here where random mating amongst an F1 population produced the same frequencies that would be seen in the following F2 and later populations.
A population if large enough, untouched and allowed to mate randomly within itself, will exist in a Hardy-Weinberg equilibrium unchanged from generation to generation. Only a disruption through external factors can effect a change, whether that be selection, migration, random drift, controlled matings, or some combination of these.
As breeders, our role is to drive that disruptive change and shift gene and genotypic frequencies in a direction that results in vastly improved animals.